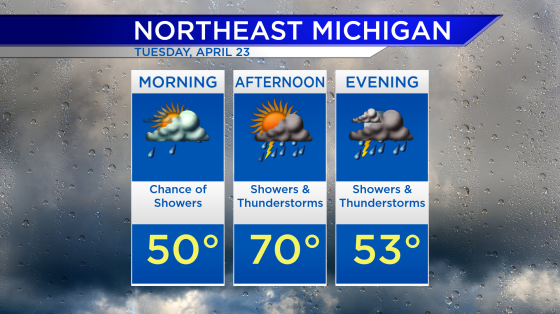
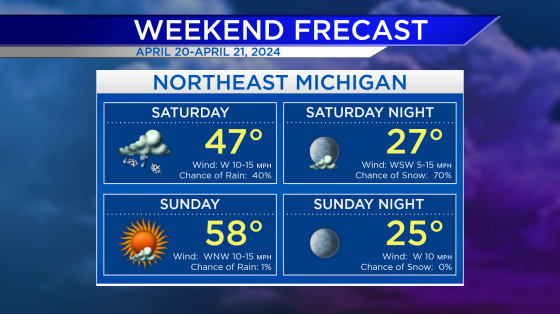
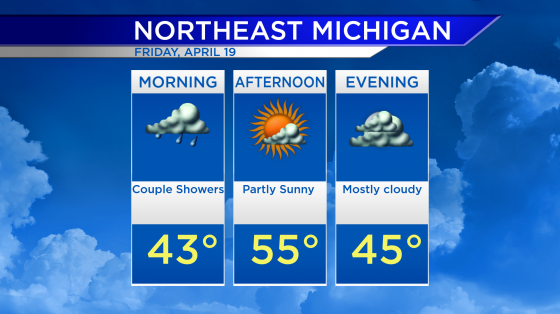
Johnson and Johnson releases Phase 3 COVID-19 trial results

NEW BRUNSWICK, N.J. — Johnson and Johnson has completed Phase 3 trials for their COVID-19 vaccine. The trial results show an 85 percent efficiency in protecting against severe coronavirus disease.
Dr Mathai Mammen, the global head of pharmaceutical research and development for Johnson and Johnson, says that what they mean by severe COVID is feeling particularly sick at home or sick enough to seek medical attention. Trial results also showed the vaccine is effective against emerging COVID-19 variants, like the one recently discovered in South Africa that has been found in the United States.
Johnson and Johnson separates themselves from current available vaccines because their vaccine requires only one dose, whereas Pfizer and Moderna require multiple. Dr. Fauci has said that the vaccine is inexpensive to create and can be mass produced. The U.S government has preordered about 100 million doses of Johnson and Johnson’s treatment, and the company expects to have 10 million available in February.
The vaccine still needs to undergo review from the Food and Drug Administration.