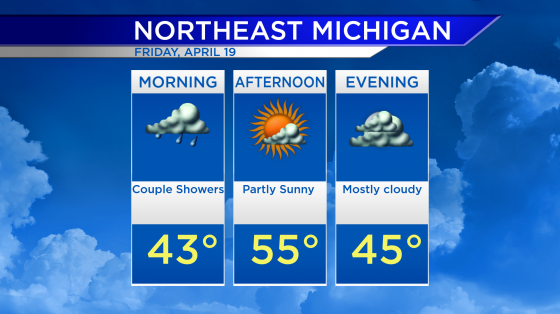
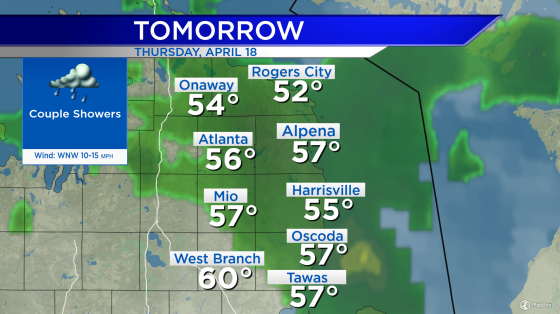
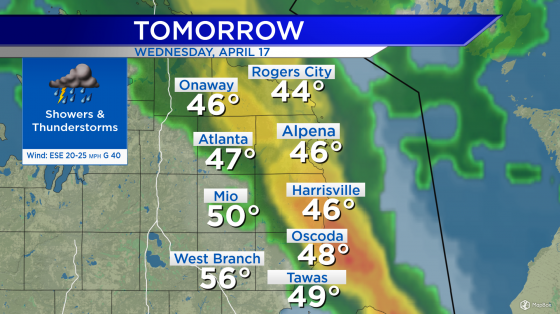
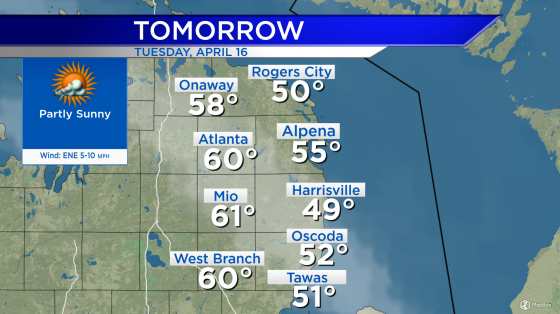
Critics at odds over Alzheimer’s drug
WHITE OAK, Md. — Critics are blasting the approval of a controversial new drug to help treat Alzheimer’s Disease.
The FDA’s Independent Advisory Committee recommended the agency reject Biogen’s drug. Seventy-eight percent of the time, the FDA aligns with their advisory committee.
When the agency ignored the advice, three committee members resigned, including Dr. Aaron Kesselheim MD, who was on CBS This Morning to explain his decision.
“The drug showed no good evidence that it worked because they’ve had important side effects,” said Kesselheim. “And then the FDA is totally switching gears over the last six months, and approving this drug, on the basis of a theory relating to the surrogate marker of amyloid plaques that we as an advisory committee back in November, were told to not consider.”
The first patient not part of a clinical trial got the drug last week. Trials show it may reduce amyloid plaque, believed to be a marker of Alzheimer’s Disease. It’s not clear whether it delays or prevents actual symptoms. The FDA released a statement saying their dataset was complex and their review of the drug was thorough.